The cover for issue 82 of Oncotarget features Figure 5B, "Kaplan–Meier survival curve of the transplanted mice," by Annesley, et al.
Here, the research team described the hematologic phenotype of a knock-in mouse model of a Wt1 mutation, described in cases of human leukemia. They crossbred Wt1+/R394W mice with knock-in Flt3+/ITD mice, and show that mice with both mutations develop a transplantable MDS/MPN, with more aggressive features compared to either single mutant mouse model.
Dr. Patrick Brown from the Sidney Kimmel Comprehensive Cancer Center & the Department of Pediatrics, at Johns Hopkins University School of Medicine, in Baltimore, MD, USA and Dr. Colleen E. Annesley from the Department of Pediatrics, at the University of Washington, in Seattle, WA, USA said, "The Wilms tumor 1 gene encodes a zinc finger transcriptional regulator that acts as a tumor suppressor in various cell types, with target genes implicated in cell differentiation, apoptosis and cell cycle regulation."
"The Wilms tumor 1 gene encodes a zinc finger transcriptional regulator that acts as a tumor suppressor in various cell types, with target genes implicated in cell differentiation, apoptosis and cell cycle regulation."
- Dr. Patrick Brown, Sidney Kimmel Comprehensive Cancer Center & the Department of Pediatrics, at Johns Hopkins University School of Medicine and Dr. Colleen E. Annesley, Department of Pediatrics, at the University of Washington
The first evidence that a WT1 mutation could be leukemogenic was a report describing a Wilms tumor survivor with WAGR syndrome, who by definition harbored a germline heterozygous deletion of the WT1 gene, and later developed acute myeloid leukemia with a new somatic WT1 mutation in the remaining allele.
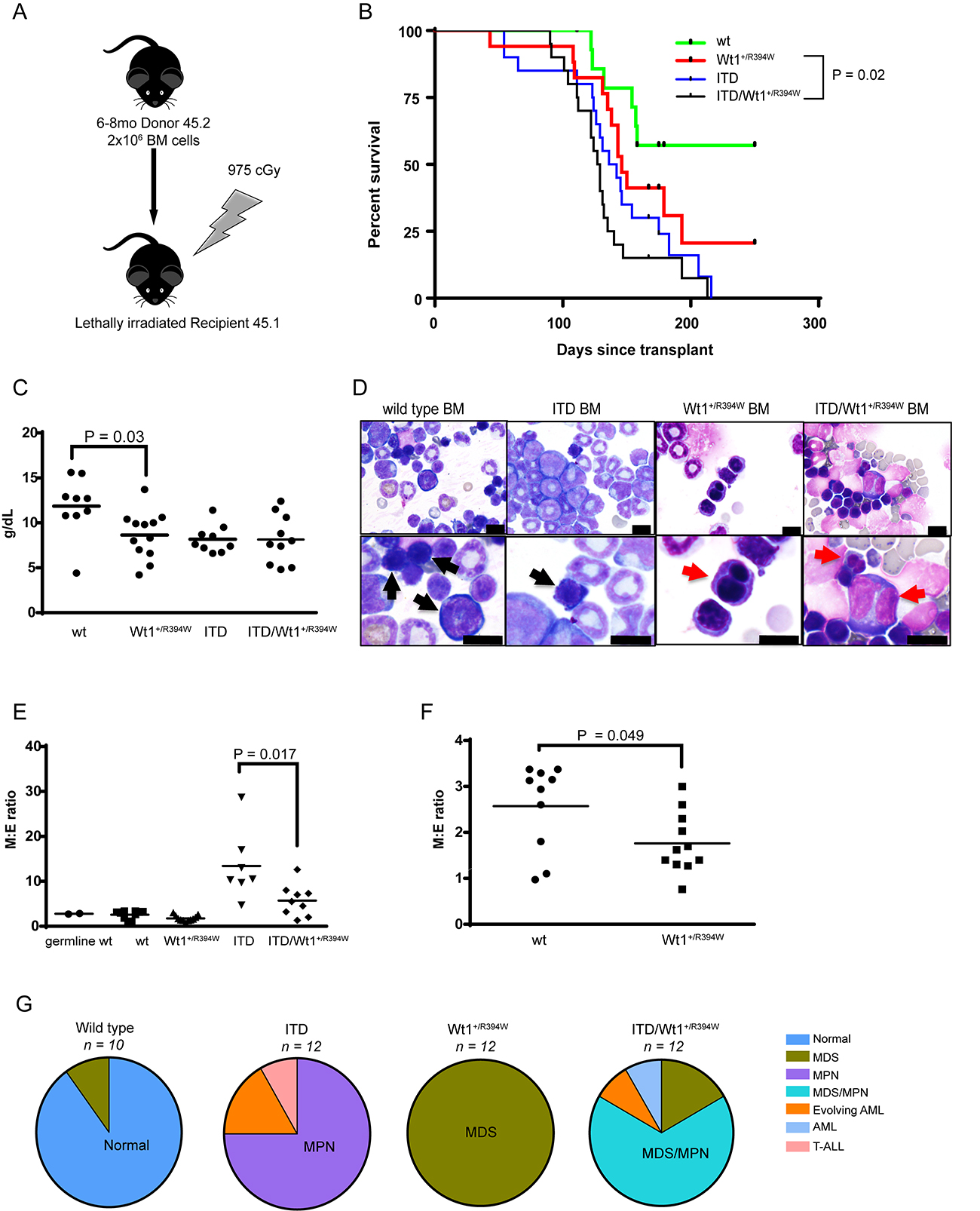
Figure 5: Hematologic characterization of a transplant model of Wt1+/R394W x Flt3+/ITD. (A) Schema of creation of transplant model. Donor mice were aged 6–8 months. (B) Kaplan–Meier survival curve of the transplanted mice: recipients of bone marrow with both mutations (ITD/Wt1+/R394W), either mutation alone (Wt1+/R394W or ITD), or wild type (wt). Survival is measured as days post-transplant; p = 0.2 for ITD vs. ITD/Wt1+/R394W; p=0.02 for Wt1+/R394W vs. ITD/Wt1+/R394W; p = 0.14 for Wt1+/R394W vs. wt. (C) Hemoglobin values of transplanted moribund mice of each bone marrow genotype at time of sacrifice. Horizontal bars represent the mean hemoglobin value for each genotype: wt 11.86 ± 1.11 g/dL, Wt1+/R394W 8.64 ± 0.76 g/dL, ITD 8.18 ± 0.51 g/dL, ITD/Wt1+/R394W 8.14 ± 0.86 g/dL. (D) Representative bone marrow cytospins from wt, ITD, Wt1+/R394W and ITD/Wt1+/R394W recipient mice. Black arrows indicate normal erythroid precursors in the wt and ITD bone marrows, and red arrows point to the dysplastic erythroid precursors in the Wt1+/R394W and ITD/Wt1+/R394W bone marrows (top row 40×, bottom row 100×; all scale bars are 10 microns). (E) Comparison of myeloid to erythroid (M:E) ratios in the bone marrow. Mean values: germ line wt 2.78 ± 0.11; transplanted wt 2.57 ± 0.3, Wt1+/R394W 1.76 ± 0.2, ITD 13.41 ± 2.91, ITD/Wt1+/R394W 5.71 ± 1.17. (F) Zoomed view comparison of M:E ratios in Wt1+/R394W versus wt transplanted bone marrow. Horizontal bars represent the mean value. (G) Distribution of hematologic phenotype by bone marrow genotype of the transplanted mice. The predominant phenotype in each genotype is highlighted. MDS = myelodysplastic syndrome, MPN = myeloproliferative neoplasm, AML = acute myeloid leukemia, T-ALL = T-cell acute lymphoblastic leukemia.
Mutations in exon 7 tend to be frameshift mutations and often occur as biallelic compound heterozygous mutations, resulting in a truncated WT1 protein and loss of the zinc finger DNA-binding domain. Recent studies of large MDS cohorts have defined WT1 mutations as an independent poor prognostic indicator, and have shown correlations of WT1 mutations with lower hemoglobin levels and a higher percentage of bone marrow blasts.
Even when accounting for the poor prognostic implication of FLT3/ITD mutations, WT1 mutations have been independently associated with treatment failure and a poor prognosis. To specifically investigate the relationship between WT1 mutations and FLT3/ITD mutations in human AML, we created a novel mouse model by cross-breeding mice with a constitutively knocked-in Flt3/ITD mutation with Wt1+/R394W mice.
The Patrick Brown/Colleen E. Annesley research team concluded, "we demonstrate here that the presence of Wt1+/R394W in the murine hematopoietic system leads to the development of MDS with single lineage dysplasia, manifested as anemia and erythroid dysplasia, and contributing to a trend in decreased survival."
Sign up for free Altmetric alerts about this article
DOI - https://doi.org/10.18632/oncotarget.26238
Full text - https://www.oncotarget.com/article/26238/text/
Correspondence to - Patrick Brown - [email protected] and Colleen E. Annesley - [email protected]
Keywords - WT1, Wilms tumor 1, myelodysplastic syndrome, AML, FLT3
About Oncotarget
Oncotarget is a biweekly, peer-reviewed, open access biomedical journal covering research on all aspects of oncology.
To learn more about Oncotarget, please visit https://www.oncotarget.com or connect with:
SoundCloud - https://soundcloud.com/oncotarget
Facebook - https://www.facebook.com/Oncotarget/
Twitter - https://twitter.com/oncotarget
LinkedIn - https://www.linkedin.com/company/oncotarget
Pinterest - https://www.pinterest.com/oncotarget/
Reddit - https://www.reddit.com/user/Oncotarget/
Oncotarget is published by Impact Journals, LLC please visit http://www.ImpactJournals.com or connect with @ImpactJrnls
Media Contact
[email protected]
18009220957x105




