Indigenous Americans: Global DNA pattern and gene expression signature in liver cancer
FOR IMMEDIATE RELEASE
2021-05-30
Oncotarget published "Global DNA hypermethylation pattern and unique gene expression signature in liver cancer from patients with Indigenous American ancestry" which reported that contrasting with this pattern, the age structure of HCC in Andean people displays a bimodal distribution with half of the patients developing HCC in adolescence and early adulthood.
To deepen the understanding of the molecular determinants of the disease in this population, the authors conducted an integrative analysis of gene expression and DNA methylation in HCC developed by 74 Peruvian patients, including 39 adolescents and young adults.
While genome-wide hypomethylation is considered as a paradigm in human HCCs, the analysis revealed that Peruvian tumors are associated with a global DNA hypermethylation.
Remarkably, the development of this HCC subtype occurs in patients with one of the four Native American mitochondrial haplogroups A-D.
Finally, integrative characterization revealed that Peruvian HCC is apparently controlled by the PRC2 complex that mediates cell reprogramming with massive DNA methylation modulating gene expression and pinpointed retinoid signaling as a potential target for epigenetic therapy.
Dr. Pascal Pineau from The Unité Organisation Nucléaire et Oncogenèse and Dr. Stéphane Bertani from The Université de Toulouse said, "Hepatocellular carcinoma (HCC), the main form of primary liver cancer, is one of the leading causes of tumor-related death worldwide."
"Hepatocellular carcinoma (HCC), the main form of primary liver cancer, is one of the leading causes of tumor-related death worldwide."
This molecular classification of HCCs relies almost exclusively on genomic data from HCC patients of North America, Asia-Pacific, and Europe.
People with Indigenous ancestry remain starkly underrepresented in cancer genomics studies, which eventually limits the usefulness of available molecular classifications for these populations.
Accordingly, the peculiar presentation of HCC in the Andean Native people of Peru supports the idea that liver cancer pathogenesis might be modulated by the patients' Indigenous American background, either through their genetic architecture or by anthropological determinants conditioning individual exposome.
Altogether, the findings uncover a major role for anthropological background in molecular oncology with the characterization of a clinically relevant molecular subtype of HCC in patients with Indigenous American ancestry.
The present study represents the first integrative genomics characterization of a molecular subtype of cancer that preferentially affects people with Indigenous ancestry.
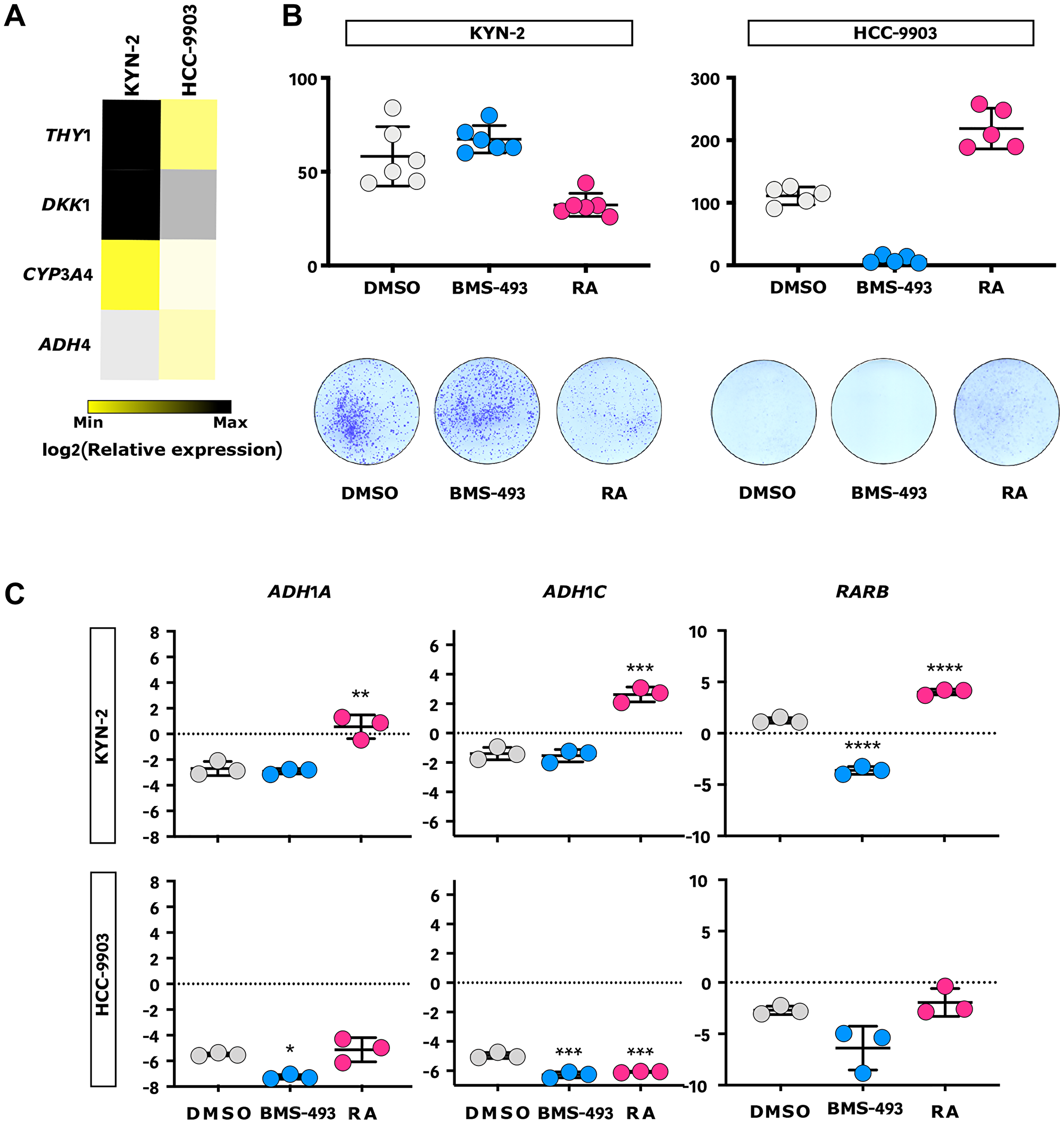
Figure 6: RA inhibits progenitor-like liver cancer cell growth in vitro. (A) Heatmap representation of the relative expression of ADH4, CYP3A4, DKK1, and THY1 progenitor-like gene markers in KYN-2 and HCC-9903 cells measured by qPCR (n = 3). (B) Outline of the effect of RA, RA inverse agonist (BMS-493), and vehicle control (DMSO) on KYN-2 (left) and HCC-9903 (right) in a clonogenic assay. Upper panel: qualitative dot plots comparing the numbers of colony-forming units (CFUs) according to the treatment allocated; Lower panel: respective illustrations of CFUs. (C) Qualitative dot plots of the relative expression of three RA-mediated genes (i.e., ADH1A, ADH1C, and RARB) in KYN-2 (upper panel) and HCC-9903 (lower panel) according to the treatment allocated, measured by qPCR (n = 3). (B, C) Blue: BMS-493; Grey: DMSO; Red: RA. Error bars represent standard deviations. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
The Pineau/Bertani Research Team concluded in their Oncotarget Research Output that the findings have implications for the development of therapeutics tailored to this newly identified molecular subtype of HCC.
Some authors have discussed the role of RA action in the prevention and treatment of MYCN-positive liver cancer stem cells, and it is apparent that a profound alteration of retinoid signaling is a crucial characteristic of Peruvian HCC.
Herein, clonogenic assays have demonstrated in vitro the ability of RA to block the proliferation of HCC cells that have a constitutive defect of the retinoid signaling pathway.
This preliminary result would represent a new avenue in molecularly-targeted therapy: the reversal and maintenance of HCC cells into a metastable innocuous hepatocyte phenotype by active, RA-mediated DNA methylation reprogramming.
The present study establishes a foundation for the dissection of the functional importance of RA-mediated epigenetic control in HCC and therapeutics tailored to patients with Indigenous American ancestry.
DOI - https://doi.org/10.18632/oncotarget.27890
Full text - https://www.oncotarget.com/article/27890/text/
Correspondence to - Pascal Pineau - pascal.pineau@pasteur.fr and Stéphane Bertani - stephane.bertani@ird.fr
Keywords - hepatitis B virus, indigenous people, integrative genomics, liver cancer
About Oncotarget
Oncotarget is a bi-weekly, peer-reviewed, open access biomedical journal covering research on all aspects of oncology.
To learn more about Oncotarget, please visit https://www.oncotarget.com or connect with:
SoundCloud - https://soundcloud.com/oncotarget
Facebook - https://www.facebook.com/Oncotarget/
Twitter - https://twitter.com/oncotarget
LinkedIn - https://www.linkedin.com/company/oncotarget
Pinterest - https://www.pinterest.com/oncotarget/
Reddit - https://www.reddit.com/user/Oncotarget/
Oncotarget is published by Impact Journals, LLC please visit https://www.ImpactJournals.com or connect with @ImpactJrnls
Media Contact
MEDIA@IMPACTJOURNALS.COM
18009220957x105
Copyright © 2025 Rapamycin Press LLC dba Impact Journals




