Oncotarget recently published "Evaluation of cancer-derived myocardial impairments using a mouse model" which reported that Myocardial damage in cancer patients is emphasized as a cause of death; however, there are not many murine cachexia models to evaluate cancer-derived heart disorder.
Using the mouse cachexia model that they established previously, the authors investigated myocardial damage in tumor-bearing mice.
When rat cardiomyoblasts were treated with mouse cachexia model ascites and subjected to flux analysis, both oxidative phosphorylation and glycolysis were suppressed, and the cells were in a quiescent state.
These results are in good agreement with those previously reported on cancerous myocardial damage.
The established mouse cachexia model can therefore be considered useful for analyzing cancer-derived myocardial damage.
"The established mouse cachexia model can therefore be considered useful for analyzing cancer-derived myocardial damage"
Dr. Yi Luo from The Nantong University and Dr. Hiroki Kuniyasu from The Nara Medical University said, "Cachexia affects 40–80% of all patients with advanced cancer, especially those with pancreatic, gastric, and esophageal cancers."
Cancer-derived myocardial impairment is characterized by morphological alterations such as left ventricular wall thinning, decreased heart volume, myocardial fibrosis, and remodeling of the left ventricle as reported in gastrointestinal, pancreatic, and non-small cell lung cancer.
The causes of cancer-derived myocardial impairment might be the effects of cancer itself, background heart disease, and influence of cancer treatments; however, they have not been given much clinical importance, and specific treatment efforts are delayed.
Various factors have been reported as the causes of cancer-derived myocardial impairment derived from the cancer itself.
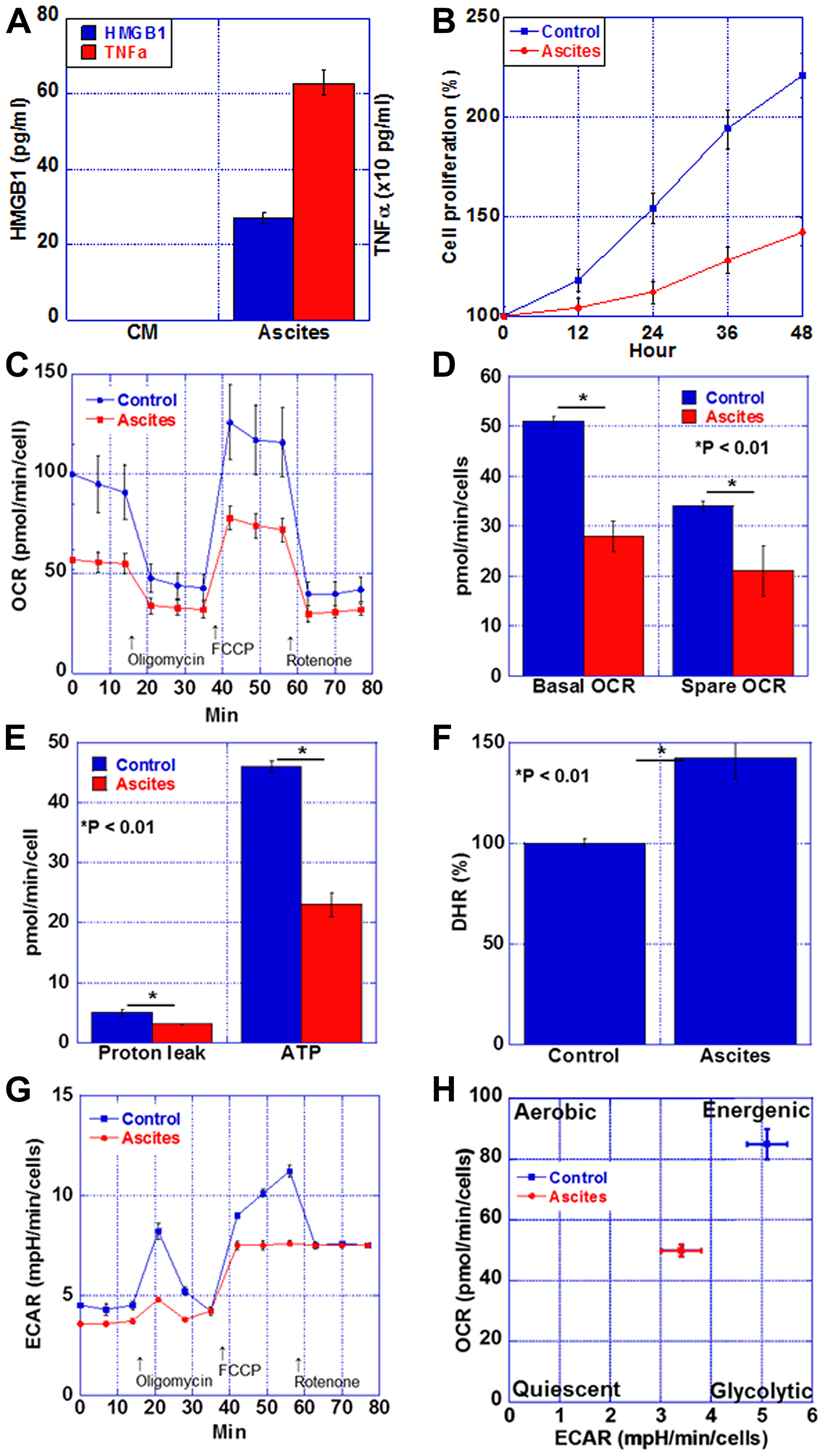
Figure 5: Energy metabolism in the myocardium of CT26-inoculated BALB/c mice. (A) Protein levels of HMGB1 and TNFα in the ascites of CT26-inoculated BALB/c mice or H9c2 cultured medium (CM). (B) Cell proliferation of H9c2 cells treated with the ascites of CT26-inoculated BALB/c mice (Ascites) or H9c2 cultured medium (Control). (C) Mitochondrial stress test (Seahorse assay) of H9c2 cells. (D) Basal OCR and spare OCR. (E) Proton leak and ATP production. (F) Oxidative stress examined by DHR. (G) Glycolytic stress test of H9c2 cells. (H) Matrix analysis of OCR and ECAR. Error bar, standard error from 3 mice. Significant differences were calculated using Student t-test. HMGB, high-mobility group box; TNF, tumor necrosis factor; OCR, oxygen consumption rate; ECAR, extracellular acidification rate; DHR, dihydrorhodamine 123.
Despite these advances in our understanding, the multifactorial mechanisms underlying cancer-derived myocardial impairment remain incompletely understood, necessitating further investigations to elucidate the molecular mechanisms and prevent myocardial damage in cancer patients.
In this study, the Oncotarget authors used the mouse cancer cachexia model that they previously established to examine the status of cancer-derived myocardial impairment reported in literature, and validate the model for studying cancer-derived myocardial impairment.
The Luo/Kuniyasu Research Team concluded in their Oncotarget Research Paper, "our established mouse cachexia model showed various myocardial changes associated with cancer cachexia such as oxidative stress in the myocardium, energy metabolism, autophagy, and inflammatory cytokines. Therefore, we propose the use of this model for future investigations of cancerous myocardial damage. We have revealed that combined feeding with laurate and glucose improves cancer sarcopenia [14]. Using this cancer-derived myocardial impairment model, we attempt to assess the effect of the diet supplemented with laurate and glucose."
Sign up for free Altmetric alerts about this article
DOI - https://doi.org/10.18632/oncotarget.27759
Full text - https://www.oncotarget.com/article/27759/text/
Correspondence to - Yi Luo - [email protected] and Hiroki Kuniyasu - [email protected]
Keywords - cachexia, myocardium, atrophy, mitochondria, oxidative stress
About Oncotarget
Oncotarget is a biweekly, peer-reviewed, open access biomedical journal covering research on all aspects of oncology.
To learn more about Oncotarget, please visit https://www.oncotarget.com or connect with:
SoundCloud - https://soundcloud.com/oncotarget
Facebook - https://www.facebook.com/Oncotarget/
Twitter - https://twitter.com/oncotarget
LinkedIn - https://www.linkedin.com/company/oncotarget
Pinterest - https://www.pinterest.com/oncotarget/
Reddit - https://www.reddit.com/user/Oncotarget/
Oncotarget is published by Impact Journals, LLC please visit http://www.ImpactJournals.com or connect with @ImpactJrnls
Media Contact
[email protected]
18009220957x105





