Corrections:
Correction: The anti-HER3 (ErbB3) therapeutic antibody 9F7-F11 induces HER3 ubiquitination and degradation in tumors through JNK1/2- dependent ITCH/AIP4 activation
Metrics: PDF 1030 views | ?
1IRCM, Institut de Recherche en Cancérologie de Montpellier, Montpellier, F-34298, France
2INSERM, U1194 Montpellier, Montpellier, F-34298, France
3Université de Montpellier, Montpellier, F-34298, France
4Department of Health and Endocrinology, University Magna Graecia of Catanzaro, Catanzaro, Italy
5Millegen SA, Labège, F-31670, France
6Biochemistry Laboratory, Instituto Dermopatico Dell’Immacolata, Department of Experimental Medicine and Surgery, University of Rome “Tor Vergata,” 00133 Rome, Italy
7Toxicology Unit, Medical Research Council, Leicester University, Leicester LE1 9HN, United Kingdom
8Institut Pasteur de Guyane, BP 6010, 97306, Cayenne Cedex, France
9GamaMabs Pharma SA, Centre Pierre Potier, ONCOPOLE, BP 50624, France
10LFB Biotechnologies, 59000, Lille, France
Published: October 11, 2024
Copyright: © 2024 Le Clorennec et al. This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This article has been corrected: Due to errors during figure assembly, the pHER3 WB of BxPC3 cells in Figure 6A has been accidentally duplicated in the pHER3 line of Figure 6B. The corrected Figure 6, obtained using original data, is shown below. The authors declare that these corrections do not change the results or conclusions of this paper.
Original article: Oncotarget. 2016; 7:37013–37029. DOI: https://doi.org/10.18632/oncotarget.9455.
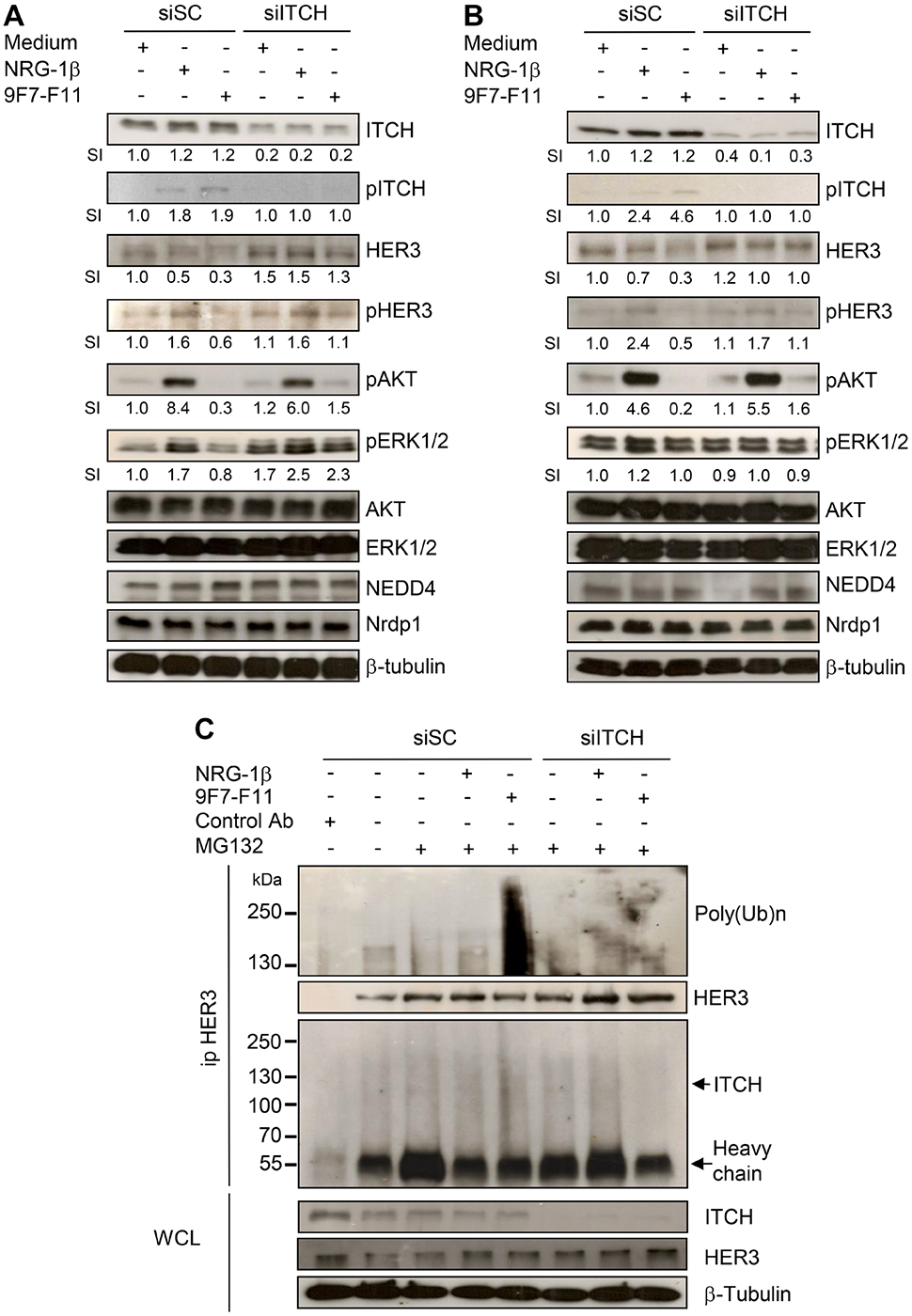
Figure 6: ITCH silencing inhibits 9F7-F11-mediated HER3 degradation and ubiquitination in cancer cells. Pancreatic BxPC3 (A) and prostatic DU145 (B) cancer cells were transfected with 10 nM Scramble Control siRNA (siSC) or the anti-ITCH/AIP4 siRNA (siITCH) for 72 hr, serum-starved and then incubated with 50 μg/mL 9F7-F11 or with 100 ng/mL NRG-1β for 4 hr. ITCH, HER3, AKT, ERK1/2, NEDD4 and Nrdp1 protein expression and ITCH, HER3, AKT and ERK1/2 phosphorylation were assessed in whole cell lysates (WCL) by western blotting. Band signal intensity (SI) was quantified with ImageJ, and β-tubulin was used as loading control. (C) BxPC3 cells were transfected with 10 nM siSC or siITCH for 72 hr, and then pre-incubated with 10 μM MG132 for 4 hr before addition of 9F7-F11 or NRG1-β for 4 hr. After immunoprecipitation with HER Ab, the HER3 ubiquitination status was analyzed by western blotting with a specific poly-ubiquitin chain antibody. HER3 and ITCH proteins were also detected by using specific antibodies.
 All site content, except where otherwise noted, is licensed under a Creative Commons Attribution 4.0 License.
All site content, except where otherwise noted, is licensed under a Creative Commons Attribution 4.0 License.
PII: 28664
