Corrections:
Correction: Cyclin E/Cdk2-dependent phosphorylation of Mcl-1 determines its stability and cellular sensitivity to BH3 mimetics
Metrics: PDF 1338 views | ?
1Department of Cancer Biology, Lerner Research Institute, Cleveland Clinic, Cleveland, OH, USA
2Department of Molecular Cardiology, Lerner Research Institute, Cleveland Clinic, Cleveland, OH, USA
3Department of Immunology, Lerner Research Institute, Taussig Cancer Institute, Cleveland Clinic, Cleveland, OH, USA
4Department of Hematology and Oncology, Taussig Cancer Institute, Cleveland Clinic, Cleveland, OH, USA
5Department of Molecular Medicine, Cleveland Clinic Lerner College of Medicine of Case Western Reserve University, Cleveland, OH, USA
6Department of Pathology, Case Western Reserve University School of Medicine, Cleveland, OH, USA
7Department of Biochemistry, Case Western Reserve University School of Medicine, Cleveland, OH, USA
Published: July 12, 2024
Copyright: © 2024 Choudhary et al. This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This article has been corrected: In Figure 4H, the first two panels in the β-actin row are accidental duplicate images. As a result, these changes alter the ratio of Mcl1/b-actin for this panel, which is presented on the graph (panel I). The corrected Figure 4 with new images for panel H and panel I, obtained using the original data, is shown below. The authors declare that these corrections do not change the results or conclusions of this paper.
Original article: Oncotarget. 2015; 6:16912–16925. DOI: https://doi.org/10.18632/oncotarget.4857
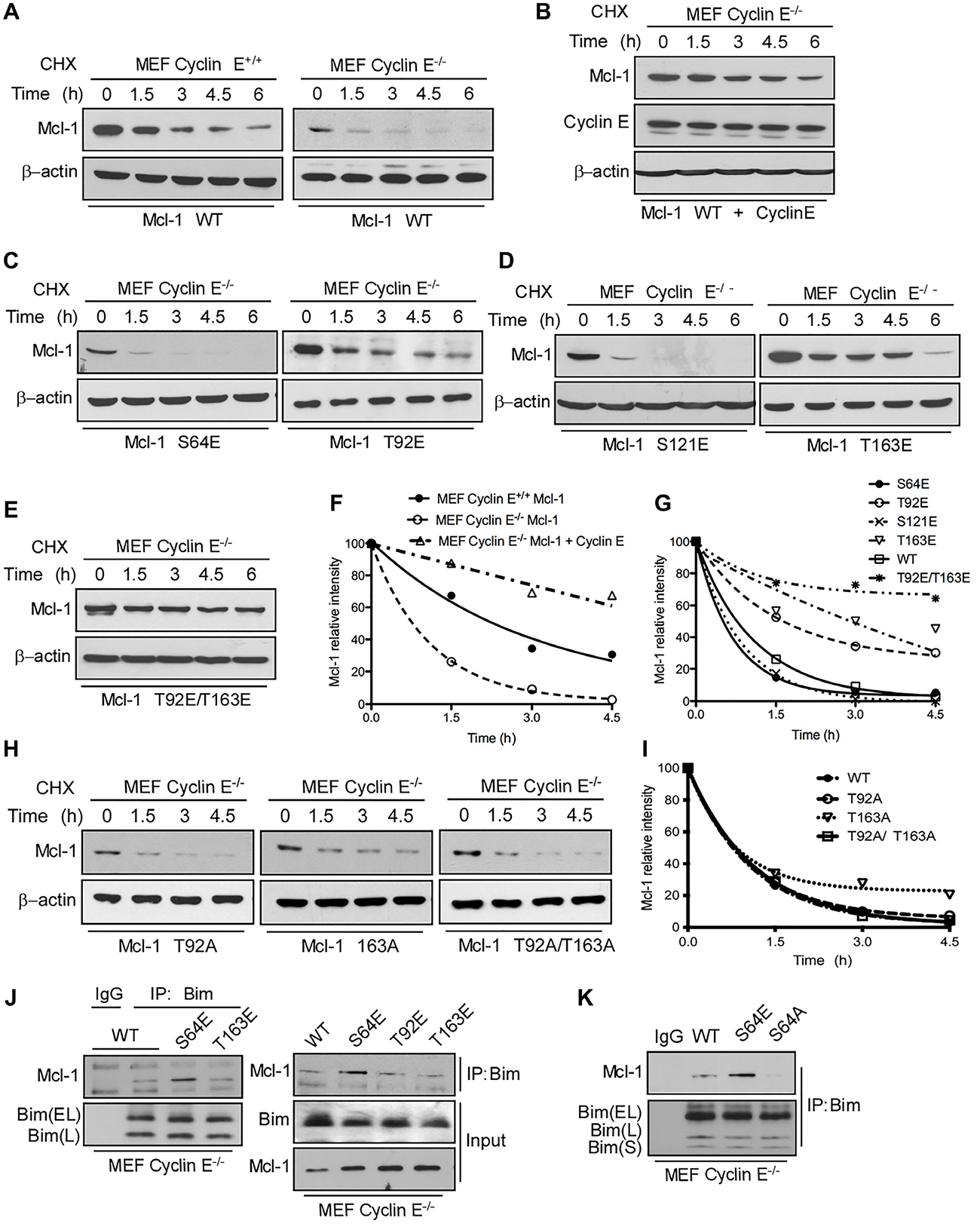
Figure 4: Mcl-1 stability and Bim sequestration is dependent on cyclin E/Cdk2. Mcl-1 protein half-life was determined by expressing WT Myc-Mcl-1 (A). individually in cyclin E+/+ and cyclin E−/− MEFs (B). together with HA-cyclin E in cyclin E −/− MEFs and then treating with cycloheximide for the indicated time, followed by immunoblotting. Immunoblot analysis of cyclin E−/− MEFs transfected with (C). S64E, T92E, (D). S121E, T163E (E). T92E/T163E (H). T92A, T163A and T92A/T163A Mcl-1 mutants and treated with cycloheximide for the indicated time. Data in (F)., (G)., (I). were quantified by ImageJ. Cyclin E−/− MEFs were transfected with (J). Myc-Mcl-1 WT, S64E, T92E and T163E (K). Myc-Mcl-1 WT, S64E and S64A. After 24 h, Bim was immunoprecipitated and its association with Mcl-1 was analyzed by immunoblotting. β-actin was used as loading control. These data are representative of three independent experiments.
 All site content, except where otherwise noted, is licensed under a Creative Commons Attribution 4.0 License.
All site content, except where otherwise noted, is licensed under a Creative Commons Attribution 4.0 License.
PII: 28593
