Corrections:
Correction: Exploratory comparisons between different anti-mitotics in clinically-used drug combination in triple negative breast cancer
Metrics: PDF 1020 views | ?
1Microscopy and Microanalysis Laboratory, Department of Cell Biology, Institute of Biological Sciences, University of Brasília, Brasília 70910-900, Brazil
2Laboratory of Medicinal Chemistry and Organic Syntesis, Exact and Technological Sciences Campus, State University of Goiás, Anápolis, Goiás 75001-970, Brazil
3Veterinary Pathology Laboratory, Faculty of Agronomy and Veterinary Medicine, Department of Veterinary Medicine, University of Brasília, Brasília 70910-970, Brazil
4Laboratory of Medicinal and Technological Chemistry, University of Brasília, Chemistry Institute, University of Brasília, Brasília 70904-900, Brazil
Published: September 28, 2022
Copyright: © 2022 Guido et al. This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This article has been corrected: In Supplementary Figure 1, there is an accidental image overlap of panel C across panel D. The corrected figure with the new panel D, obtained from the original data, is shown below. The authors declare that these corrections do not change the results or conclusions of this paper.
Original article: Oncotarget. 2021; 12:1920–1936. DOI: https://doi.org/10.18632/oncotarget.28068
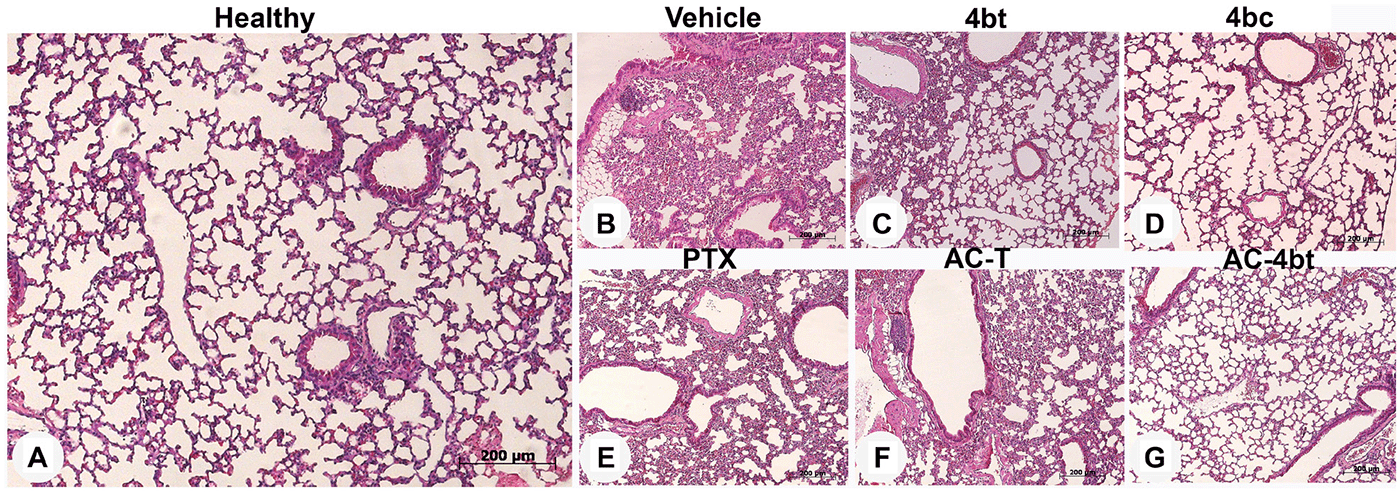
Supplementary Figure 1: Histopathological analysis of lung sections in control animal and upon monotherapy or drug combination treatments. Representative images of paraffin-embedded sections of lungs stained with H&E from the different experimental groups: (A) Healthy control, or administered intraperitoneally (i.p.) with: (B) vehicle, (C) 4bt (80 mg/Kg), (D) 4bc (80 mg/ Kg), (E) paclitaxel (20 mg/Kg), (F) Doxorubicin (10 mg/Kg) + Cyclophosphamide (100 mg/Kg) followed by paclitaxel (10 mg/Kg) (ACT) and (G) Doxorubicin (10 mg/Kg) + Cyclophosphamide (100 mg/Kg) followed by KIF11 inhibitor 4bt (AC-4bt). Metastatic foci or morphological alterations were not observed in any experimental group compared to control of healthy animals. Bars: 200 μm.
 All site content, except where otherwise noted, is licensed under a Creative Commons Attribution 4.0 License.
All site content, except where otherwise noted, is licensed under a Creative Commons Attribution 4.0 License.
PII: 28266

